|
|
[Home] - [News] |
Metallic Glass Serves as High Performance Catalyst for Hydrogen Production
Date:18-10-2016 Print
Hydrogen is one of the most promising and appealing alternative energy resources of fossil fuels to tackle the current energy crisis and environmental issues. Electrochemical water splitting with highly efficient catalyst is one of the main methods to produce hydrogen. How to improve the performance of the catalysts, including the activity and durability, is crucial for promoting the application of hydrogen energy. The currently reported catalysts including the commercial Pt/C catalyst are mainly crystalline, in which the activity is influenced by the metastable local structures. Recently, by combining experimental characterizations and theoretical computations, HU Yuan-chao et al. in Prof. WANG Wei-hua’s group in Institute of Physics, Chinese Academy of Sciences, collaborated withProf. SUN Chun-wen’s group in Beijing Institute of Nanoenergy and Nanosystems, Chinese Academy of Sciences, National Center for Nanoscience and Technology, and Prof. GUAN Peng-fei’s group at Beijing Computational Research Center,dis
covered that metallic glass (MG) could be highly efficient and self-stabilizing catalyst for water splitting.
Metallic glasses are amorphous materials fabricated by rapidly quenching the high-temperature melts. The disordered structure gives rise to their outstanding mechanical and physical properties, such as high strength, excellent corrosion resistance and superior soft magnetism etc. In addition, this latest research shows the promising potential of metallic glasses as functional materials to catalyze electrochemical reactions. Moreover, the underlying atomic-scale mechanism of the excellent comprehensive performances of the amorphous alloys was unveiled, which provides new approaches for designing novel catalysts with better performance.
This study entitled “A Highly Efficient and Self-Stabilizing Metallic Glass Catalyst for Electrochemical Hydrogen Generation” was published on Advanced Materials.
The study was supported by the National Science Foundation, the Ministry of Science and Technology of China, the Chinese Academy of Sciences and Beijing Computational Research Center.
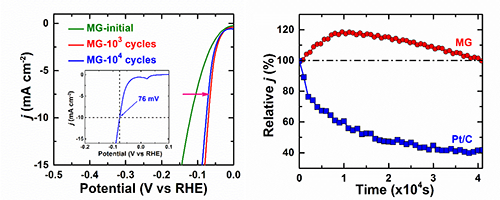 |
| Fig.1Polarization curves of the MG catalyst (left) and comparison of durability of the MG and commercial Pt/C catalysts (right). (Image by Institute of Physics) |
 |
| Fig.2 Performance of the MG catalyst compared to more than 100 reported catalysts. (Image by Institute of Physics) |
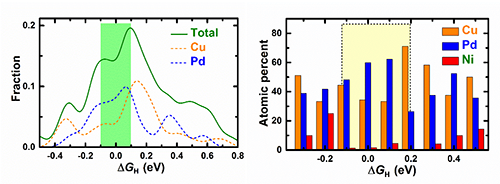 |
| Fig.3(left) Free energy ΔGH distribution of HER on different adsorption sites on the amorphous surface. Chemical environment of adsorption sites withvarious ΔGH (right). (Image by Institute of Physics) |
Contact:
Institute of Physics
WAN;G Wei-hua
Email:whw@iphy.ac.cn
Key word:
Metallic glass; electrocatalyst; hydrogen evolution reaction;
Abstract:
A multicomponent metallic glass with highly efficient and anomalous durability for catalyzing water splitting is reported. The outstanding performance of the MG catalyst contributed by self-optimized active sites originates from the intrinsic chemical heterogeneity and selective dealloying on the disordered surface; thus, a new mechanism for improving the durability of catalysts is uncovered.